
Properties
Silicon is a crystalline semi-metal or metalloid. One of its forms is shiny, grey and very brittle (it will shatter when struck with a hammer). It is a group 14 element in the same periodic group as carbon, but chemically behaves distinctly from all of its group counterparts. Silicon shares the bonding versatility of carbon, with its four valence electrons, but is otherwise a relatively inert element. However, under special conditions, silicon be made to be a good deal more reactive. Silicon exhibits metalloid properties, is able to expand its valence shell, and is able to be transformed into a semiconductor; distinguishing it from its periodic group members.
Where Silicon is Found
27.6% of the Earth's crust is made up of silicon. Although it is so abundant, it is not usually found in its pure state, but rather its dioxide and hydrates. \(SiO_2\) is silicon's only stable oxide, and is found in many crystalline varieties. Its purest form being quartz, but also as jasper and opal. Silicon can also be found in feldspar, micas, olivines, pyroxenes and even in water (Figure 1). In another allotropic form silicon is a brown amorphous powder most familiar in "dirty" beach sand. The crystalline form of silicon is the foundation of the semiconductor age.
Figure 1: Sand is an easy to find silicon deposit
Silicates
Silicon is most commonly found in silicate compounds. Silica is the one stable oxide of silicon, and has the empirical formula SiO2. Silica is not a silicon atom with two double bonds to two oxygen atoms. Silica is composed of one silicon atom with four single bonds to four oxygen molecules (Figure 2).
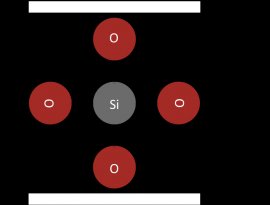
Figure 2: The net charge of silica is minus 4
Silica, i.e. silicon dioxide, takes on this molecular form, instead of carbon dioxide's characteristic shape, because silicon's 3p orbitals make it more energetically favorable to create four single bonds with each oxygen rather than make two double bonds with each oxygen atom. This leads to silicates linking together in -Si-O-Si-O- networks called silicates. The empirical form of silica is SiO2 because, with respect to the net average of the silicate, each silicon atom has two oxygen atoms.
Figure 3: This is a representation of the tetrahedral silica complex
The tetrahedral SiO44- complex (see Figure 3), the core unit of silicates, can bind together in a variety of ways, creating a wide array of minerals.
Neosilicates
In nesosilicates the silicate tetrahedral does not share any oxygen molecules with other silicate tetrahedrals, and instead balances out its charge with other metals. The core structure of neosilicate is simply a lone tetrahedral silica unit (Figure 4). The empirical formula for the core structure of a neosilicate is SiO44-.
Figure 4: The core of a neosilicate
Neosilicates make up the outer fringes of a group of minerals known as olivines.
Sorosilicates
In sorosilicates two silicate tetrahedrals join together by sharing an oxygen atom at one of their corners. The core structure of a sorosilicate is a pair of silica tetrahedra. (see Figure 5)
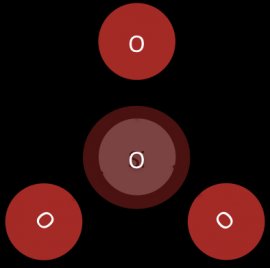 Figure 5: The core of a sorosilicate
Figure 5: The core of a sorosilicate
The mineral thortveitie is an example of a sorosilicate complex.
Cyclosilicates
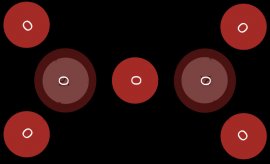
In cyclosilicates three or more silica tetrahedrals share two corners of an oxygen atom. The core structure of a cyclosilicate is a closed ring of silica tetrahedra. (see Figure 6)
Figure 6: The core of a cyclosilicate
The mineral beryl is an example of a cyclosilicate complex.
Inosilicates
Inosilicates are complexes where each tetrahedral share two corners with another silica tetrahedral to create a single chain (see Figure 7) or three corners to create a double chain (Figure 8). The core structure of an inosilicate is either an infinite single or double chain of silca tetrahedrals.
Figure 7: The core of a single chain inosilicate
The mineral group pyroxenes are examples of single chain inosilicates.
The core of a double chain inosilicate
Figure 8
The mineral amphibole is an example of a double chain inosilicate.
Phyllosilicates
Phyllosilicates are silica complexes where each tetradedral shares three corners and creates a sheet of silicon and oxygen. (see Figure 9) The core complex of a phyllosilicate is an infinite sheet of connected silica tetrahedrals.
The core of phyllosilicate
Figure 9
The mineral talc is an example of a phyllosilicate complex.
 Tectosilicates
Tectosilicates
Tectosilicates are three dimensional silicate structures. The core structure of a tectosilicate is an infinite network of connected silica tetrahedrals. (see Figure 10)
The 3d core of tectosilicate
Figure 10
The mineral quartz is an example of a tectosilicate complex.
Although many silica complexes form network covalent solids, quartz is a particularly good example of a network solid. Silicates in general share the properties of covalent solids, and this affiliated array of properties makes them very useful in modern day industry.
Silanes
Silanes are silicon-hydrogen compounds. Carbon-hydrogen compounds form the backbone of the living world with seemingly endless chains of hydrocarbons. With the same valence configuration, and thus the same chemical versatility, silicon could conceivably play a role of similar organic importance. But silicon does not play an integral role in our day to day biology. One principal reasons underlies this.
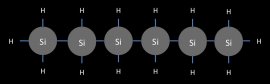
Like hydrocarbons, silanes progressively grow in size as additional silicon atoms are added. But there is a very quick end to this trend. The largest silane has a maximum of six silicon atoms. (see Figure 11)
The largest silane, hexasilane
Figure 11
Hexasilane is the largest possible silane because Si-Si bonds are not particularly strong. In fact, silanes are rather prone to decomposition. Silanes are particularly prone to decomposition via oxygen. Silanes also have a tendency to swap out there hydrogens for other elements and become organosilanes. (see Figure 12)
The organosilane, dichlorodimethylsilane
Figure 12
Silanes have a variety of industrial and medical uses. Among other things, silanes are used as water repellents and sealants.
Silicones
Silicones are a synthetic silicon compound, they are not found in nature. When specific silanes are made to undergo a specific reaction, they are turned into silicone, a very special silicon complex. Silicone is a polymer and is prized for its versatility, temperature durability, low volatility, general chemical resistance and thermal stability. Silicone has a unique chemical structure, but it shares some core structural elements with both silicates and silanes. (See Figure 13)


 An optical fiber (or optical fibre) is a flexible, transparent fiber made of glass (silica) or plastic, slightly thicker than a human hair. It functions as a waveguide, or “light pipe”, to transmit light between the two ends of the fiber. The field of applied...
An optical fiber (or optical fibre) is a flexible, transparent fiber made of glass (silica) or plastic, slightly thicker than a human hair. It functions as a waveguide, or “light pipe”, to transmit light between the two ends of the fiber. The field of applied...